The engine wont start, or it turns slowly. But your battery is only two years old, and it was expensive! Dang it…the thing should work, and you should be flying right now.
You’ve been there, done that and cursed the battery. Sometime during the process of pulling the old one out and installing a new one, you probably toyed with the idea of going to an auto-supply store and buying a cheap battery; its only used for starting and you could hand-prop the plane, so why incur the expense? How different could they be?
With that as my premise, I went off to the battery manufacturers with a few basic questions: Are airplane batteries different from automobile batteries? If not, why the higher cost? If so, then how are they different, and do they require different maintenance? The short answers: Yes, airplane batteries are different; and, yes, they require different maintenance.
Into the Way-back Machine
First, let’s review the nature of the beast. Although Ben Franklin coined the term, and the first battery was built before 250 BCE, in Baghdad, the first practical battery was invented in 1859 by French physicist Gaston Plant. Today there are several variations on it with techno-names such as recombinant gas and absorbed glass mat, but at their heart, they’re still based on Plants idea.
Our friend Plant was fiddling around in the lab with lead, which seemed to be very resistant to the effects of acid. He discovered that, though the lead wasn’t eaten away as were most other metals when dunked in acid, the combination did generate a voltage. More significantly, he discovered that this battery could be discharged and then re-charged. But he was stuck at 2.2 volts per circuit. It didn’t matter if the lead plate had more surface area or a greater thickness; it was 2.2 volts per plate. We’re still there.
Raising the voltage was easily done; just add more plates and connect the positive lead on plate #1 to the negative lead of plate #2. Connect the voltmeter to the remaining poles, and you see 4.4 volts. Put six sets of plates together in this fashion, and you get 13.2 volts.
My expert on the subject of batteries, Skip Koss, at Concorde, laughed when I asked why its called a 12-volt battery if its really 13.2 volts. Ive no idea, he said. Tradition! I got a call from a guy wanting to know what was wrong with his battery; it was down to 11.5 volts and wouldn’t start the airplane. I had to tell him it was a dead battery, but I couldn’t convince him that the numbers don’t really tell the story.
Before going further, lets define a few terms and offer a couple of analogies:
Voltage (V) is the potential to do work; think of it as water pressure, measured in PSI.
Current (amps) indicates how many electrons are flowing through a conductor, measured in Amp-hour (Ah). Multiply amps times hours and you get amp-hour. Think of this as gallons per hour such as in a large river.
Power (watts) is amps multiplied by volts, measured in gallons per hour at some psi. A large, fast-flowing pipe filled with water under pressure.
Watt-hours. All of the above multiplied give you the equivalent of gallons per hour for a number of hours. Visualize a large fast-flowing river able to flow for a lot of hours.
Its about as obvious as a thumb tack on a church pew that rating a battery in amp-hours is not the best measure of the battery; watt-hours would be ideal, but the industry is stuck with Ah. Maybe thats short for Ah, well.
Trade-offs and Compromises
Plant also discovered that with plates having a greater surface area, he got more power (amperage) to turn heavy loads; with thicker plates, he got a longer discharge time. Think of the plate as a sponge for electrons; with a lot of surface area the electrons are on the surface; a thick sponge has them deeper in.
The other trade-off is in the system voltage. If you have a 1200-watt device, you need a combination of voltage multiplied by amperage: W = V x A. But as amperage goes up, the wiring gets hot. 12 volts x 100 amps = 1200 watts, and that will definitely heat the wiring. Ever notice how hot jumper cables get?
But if you raise the voltage, you can lower the amperage: 24 volts x 50 amps = 1200 watts. Now you can use smaller gauge wiring. The only problem is that 24-volt devices are more expensive.
Think on that for a minute, and you’ll see that these factors can be traded around according to the requirement. A large airplane needs to have a deep-discharge capacity to run flaps, avionics and landing gear in case the generating system quits. That means a 24-volt system (or more) and a battery with thick plates for deep discharge. (Remember that how its wired internally, not plate geometry, determines the voltage.) Every pound and dollar counts for more in a small airplane, so that 24-volt system with its expensive components and big battery is a no-go.
But a car uses a 12-volt system, so could we just use a car battery? Not a good idea. In the course of the day you’ll start your car several times. These are short-duration, high-power events that require plates with a large surface area. If you keep cranking, the car battery will quickly go flat. Do it repeatedly and the battery will become junk. For instance, put a car battery in a wheelchair, and you can expect about 30 days of use even with daily charging.
Your small airplane, though, wants only one or two events like this each day, but if the generating system quits, the battery has to keep the lights and radios running until you get the plane on the ground. Thats a requirement for light weight, lower amperage and longer duration, which means thick plates.
A boat is a similar situation, but weight doesn’t matter, so thick plates are fine, but component expense keeps it to 12 volts. A golf cart must have endurance and only needs to run one component, so it uses thick plates and up to 48 volts…you get the idea.
Its interesting to note that as cars become far more electrified, the manufacturers are contemplating a move toward higher-voltage batteries. And, of course, all the hybrid cars on the road use very high voltage. A Toyota Prius battery module puts out 273.6 volts and has a 6.5 Ah capacity. That’ll get you started.
Stacking the Deck
That, in turn, leads to the division of lead-acid batteries into two groups: deep-cycle batteries and starter batteries. Any combination of these two ends of the spectrum results in a middle ground thats not really good at either application.
Geometry can be employed to help out for either group. Total surface area is the parameter we’re looking at in large plates, so take a small plate and punch a lot of holes in it. You get a lot of surface area and light weight in a small package. Now make the plate a bit thicker, and you get deep discharge. It’s not a perfect solution, but it helps.
The battery box can help out as well. They come in two groups: flooded and non-flooded. Deep-cycle flooded cells usually have removable caps that allow you to replace any loss of electrolyte. These are on older cars and garden tractors, and they’re still available. Sealed flooded cells don’t have caps, so the electrolyte cannot be replenished. When the electrolyte level has dropped, the battery must be replaced.
Keep those configurations in mind as we jump into a bit of basic chemistry. The electrolyte in the battery is sulfuric acid (H2SO4) which, in the process of making electricity, breaks down into gaseous hydrogen and oxygen that simply blow away into the atmosphere. No Smoking signs abound in the battery shop for good reason. Whats left behind in the battery are sulfates in the liquid.
From this you can see that adding fresh electrolyte would imbalance the mixture; if you add distilled water, however, it can combine with the excess sulfate and return the mixture to a balance.
This is done automatically in the valve-regulated lead-acid (VRLA) battery. The idea is to minimize the loss of hydrogen and oxygen by sealing the battery and keeping it pressurized at 1 to 4 psi. The extra pressure helps to recombine 99% of the gases back into water and then mix with the electrolyte, keeping the mixture closer to optimum.
It’s not perfect for two reasons: One, the battery has to be vented to prevent excess pressure buildup, so there will be some losses; and two, there’s another actor on our chemical stage. Those sulphur atoms, the S in H2SO4, don’t just float around waiting to bond to water; they turn into a crystalline form called sulfates. It’s a natural part of the process, but can be avoided by keeping your battery charged. A cautionary note is in order: Not all chargers are the same; the wrong one can destroy the battery. We’ll discuss chargers in a separate article next month.
Either of these groups, deep-cycle flooded or sealed flooded batteries, will work if you’re a non-aerobatic pilot and/or never get into much turbulence. But if you’re the negative-G type, you want the liquids under control. Enter the VRLA sub-groups of gel and absorbed glass mat (AGM) batteries.
As the name implies, in a gel battery the electrolyte has been thickened to a gel with an agent such as fume silica. No splash, just dash. AGM batteries go low-tech and just soak up the liquid with a sponge made of fiberglass.
Neither of these will leak if the case is cracked, and both are within the VRLA group, so they recombine the hydrogen and oxygen. You begin to see, then, that the technologies can be combined in different ways, making it quite hard to absolutely classify an individual battery.
There’s another advantage to gel and AGM batteries: They’re tougher. Pure lead is soft with little mechanical strength, so its usually alloyed with antimony. However, antimony also increases the self-discharge rate to as much as 40% per month. With a gel or AGM battery we have the internal construction for supporting higher purity lead, so that discharge rate drops to as low as 2%. The result is that these batteries can be left in your airplane without being charged for quite a bit longer than your typical car battery.
Putting It All Together
Add this last bit of knowledge to the selection process and we see that, for our smaller airplane, we want 12 volts for low-cost components; we only start it once or twice a day so we don’t need large plates; it needs thick plates for long-duration use; it should be sealed to guarantee a non-leaking electrolyte; and it will sit sometimes for a month or more, so it has to have a low self-discharge rate. Those are not the requirements placed on a car or tractor battery, so it narrows our choice to AGM or gel types designed for aircraft. With one more factor, capacity, brought on stage, you’ll see that out of this morass of technology there appears a clear winner.
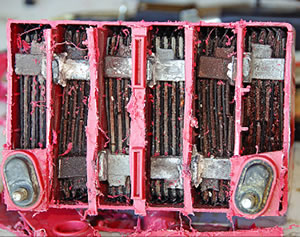
Total capacity and available capacity are two terms not often referred to, but they’re important for aviation batteries. Total capacity is, as the name says, how much power is in the box. Think Ah or, better still, watt-hours. Available capacity, on the other hand, is a function of how fast you can convert chemistry into electricity. This value will always be less than total capacity.
Heres where the let it rest advice after cranking for an abnormal period comes in: You have to let the battery chemistry catch up, and the starter motor and wires cool down. You can use that knowledge to help you on a cold day. Cold weather slows the chemistry and reduces the cranking speed. Warm up a cold battery by turning on the lights and radios for a few minutes. Turn them all off and then start the engine with a battery thats had a bit of warming.
This behavior was noticed back in 1897 by a German scientist named W. Peukert. He described the charging and discharging behavior of batteries in some rather neat equations. No, I wont go into them. I mention it only so you electrical types can go get the details. Just remember that AGM cells can be charged at higher rates than gel or flooded batteries.
The light shineth! Absorbed glass mat is the battery for airplanes.
Right now you’re probably wondering why Ive not discussed exotic batteries. The answer is another factor called conversion efficiency, which describes the efficiency of the conversion (not the speed) between chemical and electrical energy. Simply put, the lower the internal resistance of a battery, the better the conversion efficiency.
Monsieur Plants concept of lead-acid is still with us because it sits up there at about 90% conversion efficiency compared to NiCad at 65% and alkaline at 60%.
Shelf Life: Should You Care?
Any battery will self-discharge and, in the process, some of those sulfates will crystallize on the plates. Charging knocks the sulfates off in a fine rain of powder to the bottom of the case. The longer it goes without a charge, the thicker the layer, so that when it does get charged, some of the lead goes with the sulfate. The sulfate is an insulator, but now, with the entrained lead, we have a conductor that, when the trash pile is deep enough, shorts out the cells. You now have a battery somewhat akin to Monty Pythons parrot: Hes not dead, hes just resting!
Older style batteries addressed this problem by having a deep case that would allow the trash to pile up. AGM batteries encase the plates and mats in sleeves as part of the structural bracing; because they are pure lead, they don’t self-discharge so quickly and don’t build up the sulfates. Therefore, they don’t need the trash bin, and the battery is smaller and has a much longer shelf life.
One other problem here, though. Sulfates start as a spongy white coating that in time hardens to a solid insulator, reducing battery power. The buildup will eventually insulate enough surface that the area simply cannot be charged, or when the battery can no longer reach 80% of the capacity for which is was originally rated, its hasta la bye bye.
The Maintenance Regimen
You’ve heard the myth that if you let your car battery go dead three times, its time to replace it. Sometime those myths are true. In this scenario, the assassin is not always sulfates; instead, its physical damage caused by unregulated charging.
Envision an empty milk carton being filled by a fire hose. Thats what happens when an unregulated battery charger is attached to a dead battery. Instead of blowing out the bottom of the milk carton, the influx of electrons overheats the plates, warping and cracking them.
Additionally, the electrolyte can boil, and if the battery is sealed, the pressure buildup may warp the case. All of this is obviously not good and, just as obviously controllable.
One last bit of advice: Don’t just toss a battery of any size into the junkyard. Drop it off at the battery store; old batteries are valuable and recyclable.
The subject for next month: battery chargers. They’re not all the same, and chances are yours is a battery killer.