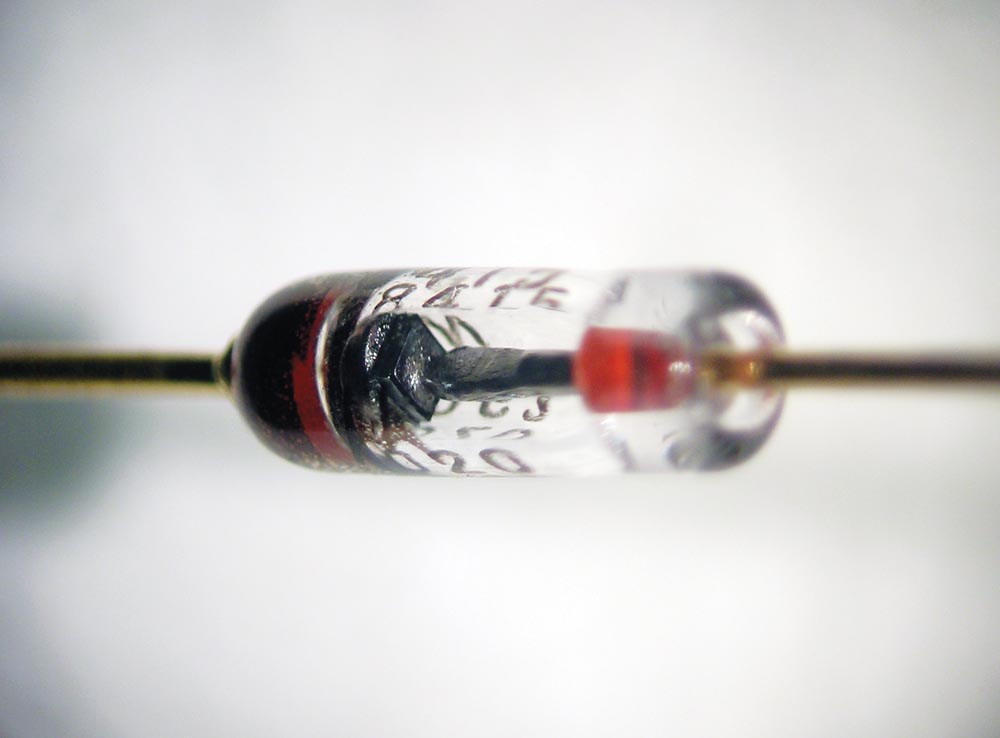
This diode is similar to the radar detector germanium diodes used in WW-II. (Photo: John Maushammer [Wikipedia, CC BY-SA 2.5, https://creativecommons.org/licenses/by-sa/2.5])
This started out to be a discussion of diodes and how they work, but for me to adequately explain them, I must confess to an electrical untruth that has been played on you since Ben Franklin (late of Philadelphia) made his pronouncement in 1752. He proclaimed that electricity flowed from the most positive point in the circuit to the most negative point. This after he knocked himself on his keester with his sparking kite experiment, which may explain this incorrect pronouncement.
J. J. Thomson (who discovered the electron in 1897), Robert Millikan (who found the negative charge on the electron), and Thomas Edison (who used these discoveries to make the first diode), found exactly the opposite, that electricity flows from the most negative point in the circuit to the most positive point.
Yep, fellers, you all think your radios work from the positive battery bus through the radio to the metal airframe or negative bus. Sorry, those electrons are coming from the negative pole of your battery up through the radio and out into the positive bus. In light of this, I think I owe you an answer to Santa Claus and the Easter Bunny as well.
But most of us (yours truly stands accused) work our theory with the positive bus providing the power to the device into the negative bus. It ain’t so, Joe. But it makes it easier for us to understand how things work. We call this “conventional” current flow since that’s how most of us learned it all the way from vacuum tubes to microprocessors that use a “positive supply” to power the circuit.
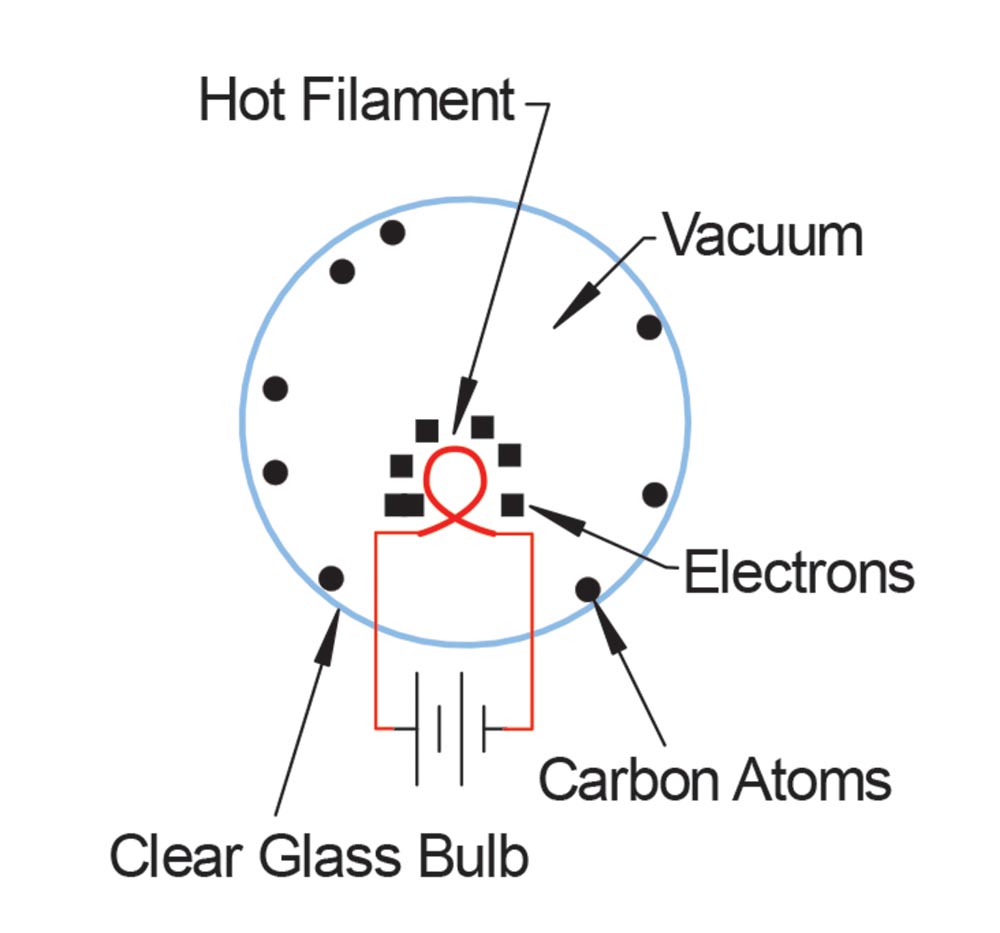
Back when Edison was perfecting the light bulb invented by Joseph Swan, he used a filament made from sewing thread dipped in coal dust. In a vacuum bulb, the coal dust would glow when a current was passed through it, but some of the carbon atoms in the coal would burn off and plate themselves out on the relatively cool glass vacuum bulb. This darkened the glass, which reduced the light output, which made the light bulb worthless. Edison reasoned, incorrectly, that he might be able to attract these neutral carbon atoms with electricity, so he put a small metal plate in the vacuum bulb and hooked up a battery between the filament and the plate.
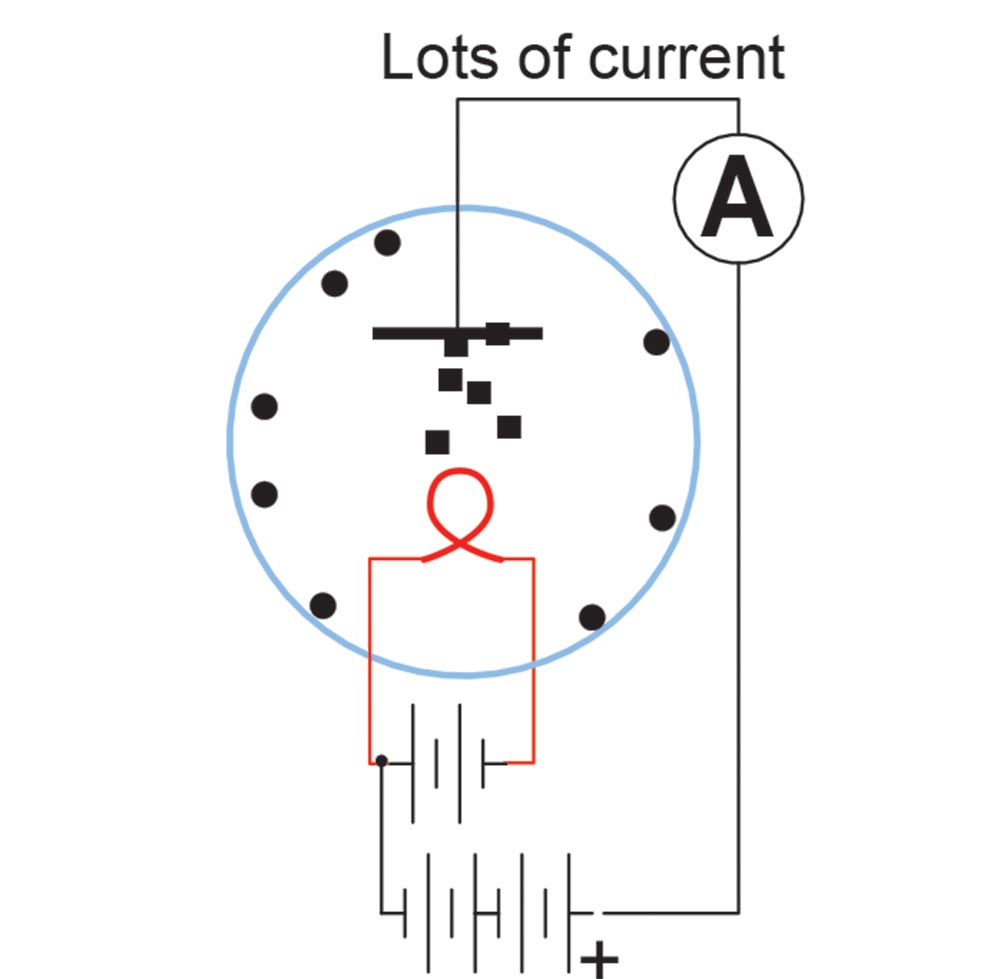
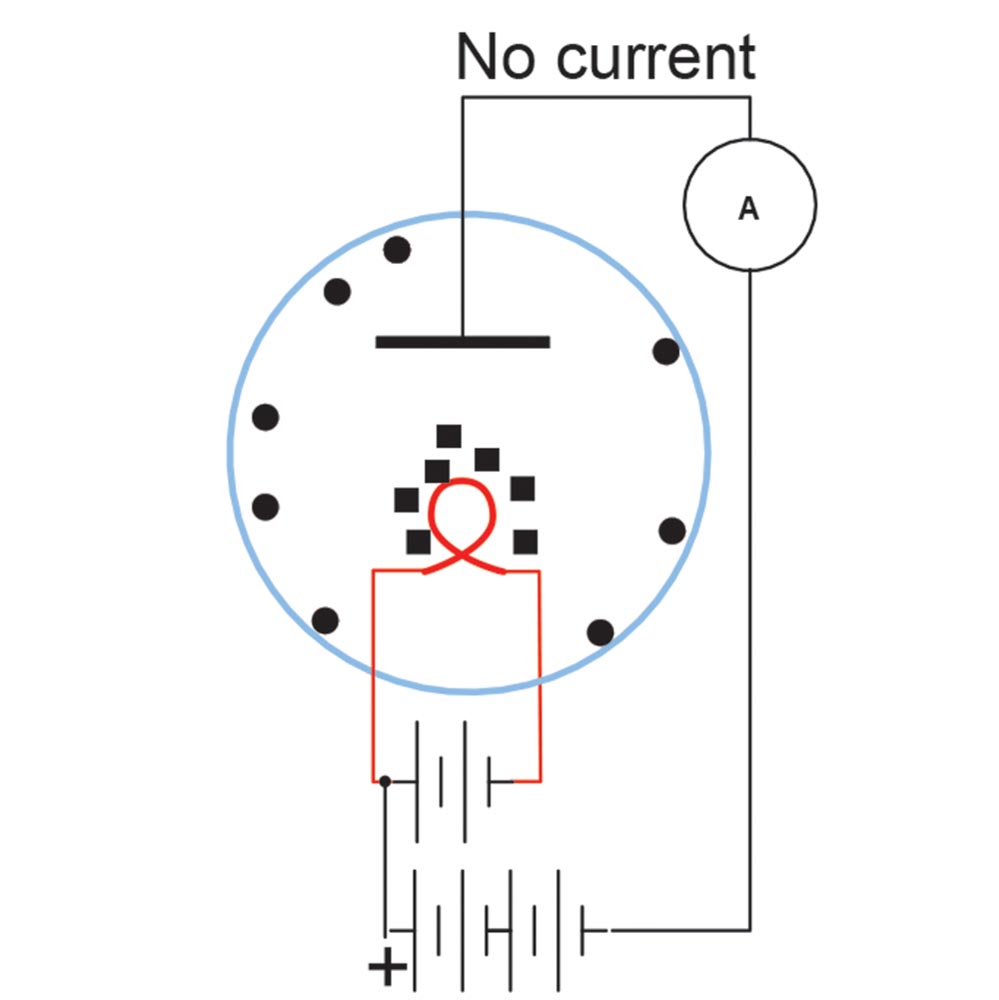
It didn’t work. The neutral carbon atoms were not at all attracted to the metal plate, no matter which way he hooked up the battery. What he did find was that with the positive lead of the battery attached to the plate and the negative lead to the filament there was a current flow, but when he reversed the battery the current went to zero. He noted this in his records and tossed the notebook onto a shelf. He had invented the diode but didn’t realize it. You see, when any conductor gets to the incandescent point, it breaks apart some of the internal atoms and sends the electrons in the atom into the space around the filament. The positive pole of the battery attracted these electrons onto the plate but when the battery was reversed, the negative pole repelled the electrons and current flow stopped.
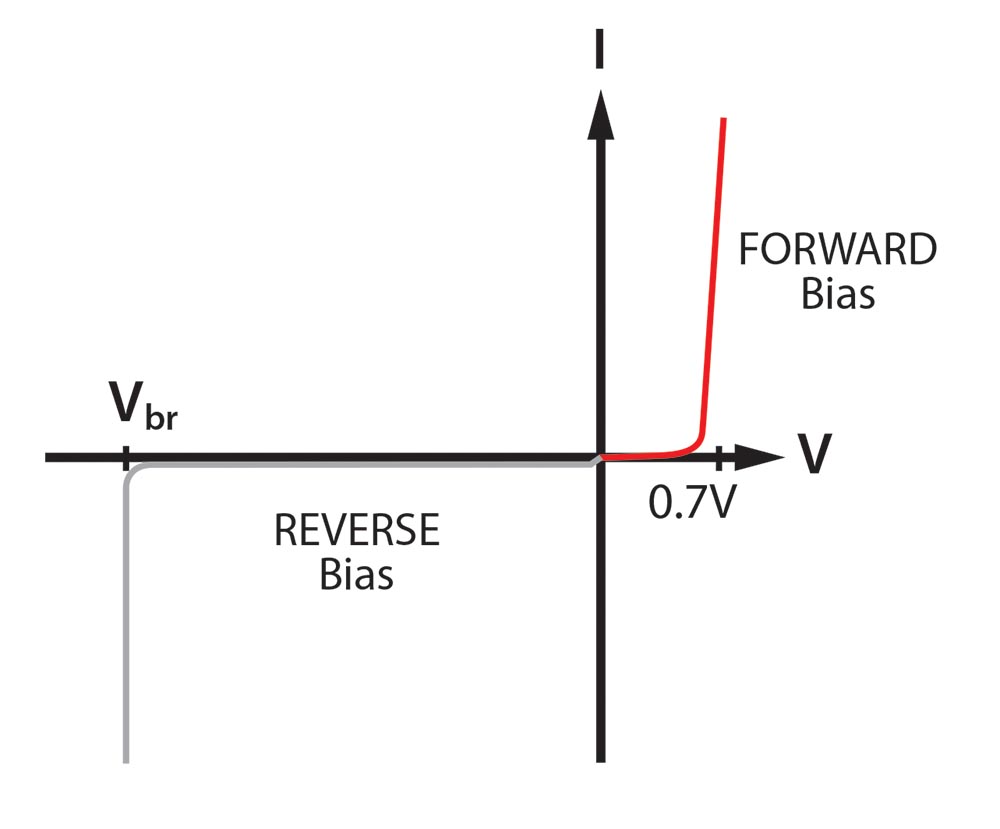
The voltage versus current curve of a silicon diode. The reverse voltage curve is something that we will explore in future columns.
Thus the fundamental property of any diode: they conduct in one direction and are an insulator in the other direction. They’re an electronic version of the hydraulic check valve if you like.
John Fleming in England rediscovered the “Edison effect” and thus began the vacuum tube era in radio, which set the stage for some really interesting homebrew experiments. In the early 1910s, when radio was just in its infancy, some radio geeks found that galena (lead sulfide) made a pretty good diode. (Fun fact: Galena, Kansas, was the source for a lot of this mineral.) Other experimenters found that germanium would work well, but was very temperature sensitive. Hundreds of crystals were used, some better than others. And thus was born the “crystal set” radio.
But it took a war (WW-II) and the invention of radar to advance the state of diodes. Germanium was the element of choice, and the venerable 1N34 (perfected in the 1N270) was the diode of choice in those days for a sensitive detector diode for the US and Britain’s radars looking for Heinkel bombers coming across the channel.
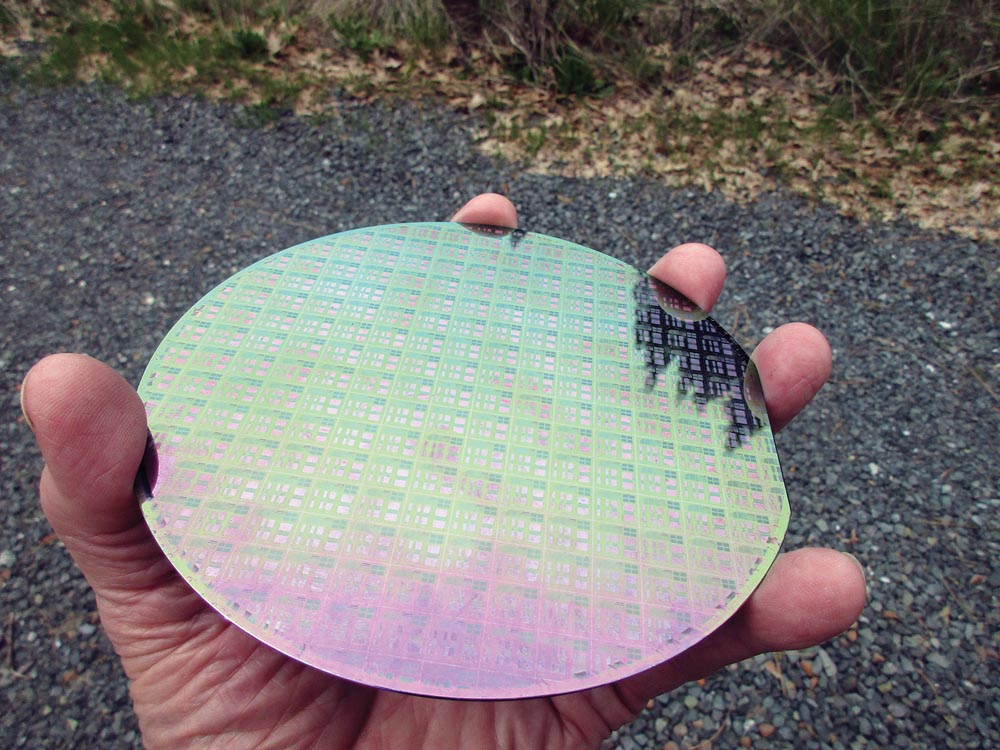
Here is how we make a regular old diode today. Most diodes are made of silicon, so I’ll show you the evolution of a silicon diode. You take an elemental chunk of silicon. How do you get silicon, you ask? Beach sand is silicon dioxide. Melt the sand, dissolve the oxygen, and what is left is impure silicon. Make a “candle” of the molten silicon by dipping a perfect silicon seed into the molten silicon and drawing it out. Let it harden. Dip it in again. Let it harden. Lather, rinse, repeat. Then melt your candle down and do it again, and again, and again, until you get a perfectly pure silicon rod about 6 inches in diameter.
Then you slice it dime-thin and “dope” it to create anode and cathode areas and you have a diode. Doping it involves introducing other elements into one side of the silicon or the other to create the cathode on one side and the anode on the other side. You get about a million diodes from a single 6-inch silicon “wafer.”
So let’s see what this diode really does. To do so, we will use the currently popular 1N4148 silicon diode. If I connect the negative terminal of a power supply to the cathode of the diode and the positive terminal to the anode of the diode (with appropriate voltmeters and ammeters to measure the current), we find that just about up to a power supply voltage of 0.4 volts or so, those little electrons sitting on the cathode have absolutely no interest in jumping over into the anode area. However, at about 0.5 volts, some of the more adventurous electrons are drawn from their negative buddies in the cathode to their positive counterparts in the anode; at 0.6 volts, even the marginally timid electrons are going over to the anode, and by 0.7 volts all the electrons are jumping over to the anode—the diode is in full conduction.
Applying voltage in the reverse direction from the above, you can increase the voltage to over 75 volts and only one electron in a billion (1 nanoampere) will somehow sneak across from one side to the other (there is always one in the crowd, isn’t there?)
Thus we end Lesson 1 of Diodes 101 to be continued in a few months. If you think this is black magic, wait until we get to Transistors 101. See you next month for antenna basics. Until then… Stay tuned…